Links Between the Gut Microbiome and Chronic Stress
Bi-directional Connections Between Gut Health and Adverse Experiences
Introduction
A friend from my School days, who I’d not seen in a few years, visited me last week, and I mentioned how healthy he looked. He related to me that it was because he had implemented some strategies for improving his gut microbiome, after reading some posts I had written on the topic on facebook. Ever since, he reports he has been feeling really well and felt very clear headed. Based on this testimonial, I thought it would be worth collecting together the stuff I had written on the topic here.
Before we begin, I want to clarify where I am coming from on this. I am not a believer in “single extrinsic causes” of chronic illnesses (apart from perhaps chronic stress). One can find many articles claiming that a specific bacteria, fungus, virus, toxin, or nutritional deficit is the root cause of all cases of chronic condition like Parkinson’s Disease (and they can’t all be right)! I just don’t buy that.
Instead, I subscribe to the threshold theory of chronic disease, in which a combination of stressful events and negative impact factors add up over a lifetime, until there is a “straw which breaks the camel’s back”, causing symptoms to become life limiting and debilitating.
In this model, different people will have different combinations of impact factors, which all add up until they manifest as a personal set of symptoms.
of the substack, in his recent podcast with Ben Ahrens, provides a very clear explanation of this. He calls it the “Jenga” theory, where each impact factor is like taking a brick out of a Jenga tower, until eventually so many bricks have been removed that the tower falls down. While many people and doctors will only focus on the last brick that was removed as being the root cause of symptoms, it is the entire history and the combination of all the every brick that led to tower falling.Likewise, to get fully better, it is not enough to address only the final impact factor, but all the bricks need putting back. So just like there is no single cause, there is no “single solution”. All the historical impact factors need addressing.
Hence, to be clear, here I am not claiming that the gut microbiome is the root cause of chronic disease, nor that addressing is a cure-all. Instead, the microbiome is, for many, an important factor in symptom severity, and may be part of the holistic solution. It is not even a case of “which came first”, or chicken-and-egg, when comparing gut health with the other contributing factors, because these issues all tend to “co-evolve”, as we shall see.
Connections with Adverse Childhood Experiences
In my previous article,
we explored how stressful episodes and trauma in early life significantly increases the chances of chronic disease in adulthood.
According to the science article “The Microbiome: Development, Stress, and Disease”, disruptions of the microbiome, due to the early adversity, could be one of the factors which increases the propensity of a chronic illness in later life.
“The importance of the early life gut microbiota in shaping later health outcomes also is emerging. Results from preclinical studies indicate that alterations of the early microbial composition by way of antibiotic exposure, lack of breastfeeding, birth by Caesarean section, infection, stress exposure, and other environmental influences - coupled with the influence of host genetics - can result in long-term modulation of stress-related physiology and behaviour.”
“The gut microbiota has been implicated in a variety of stress-related conditions including anxiety, depression and irritable bowel syndrome, although this is largely based on animal studies or correlative analysis in patient populations. Additional research in humans is sorely needed to reveal the relative impact and causal contribution of the microbiome to stress-related disorders.”
Reading further, the impacts of such gut microbiome imbalances from early ages also go some way to explaining the anxious behaviours seen in the adults who have experienced developmental trauma, such as the well documented alterations to personality traits, and trauma Survival Styles, see:
However, the arrows point the other way too, and the stressful behaviours, trauma Survival Styles, and over-activated left brain hemisphere ways of attending to the world, may keep the body in states in which the establishment of healthy microbiome balance may be difficult. In other words, the environment of the body under constant stress or fight/flight/freeze Nervous System states may not be optimal for some [good] bacteria, but may be conducive to other [bad] ones.
For example, bacteria are known to be affected by electric fields, and the electrical states of the Nervous System may have direct impacts on them. Also, the ingredients of the “chemical soups” the microbiota swim in will have important implications for various microbiome populations, and hence the state of the endocrine system will have some influence.
Another possibility is the effect of the acidity of the body. People suffering from trauma and chronic conditions tend to significantly over-breath, leading to low levels of CO2 in the blood, which causes the blood to shift to a more alkaline state. Also, we tend to have frozen diaphragms, and hence the natural role of the diaphragm in constantly massaging the gut, and thus the stirring of the environment of the microbiome, is greatly diminished.
Connections with the Vagus Nerve and the Brain
Another very important science article is “The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis”. It supports my view above that imbalances in gut microbiome, are just as much an effect of Nervous System dysregulation/chronic illness as a cause. In particular, this draws the important bi-directional arrows between Vagus Nerve dysfunction and gut biome disruption, while pointing to chronic stress/trauma being the principle causative mechanisms for both.
“The microbiota, the gut, and the brain communicate through the microbiota-gut-brain axis in a bidirectional way that involves the autonomic nervous system. The vagus nerve (VN), the principal component of the parasympathetic nervous system, is a mixed nerve composed of 80% afferent and 20% efferent fibers. The VN, because of its role in interoceptive awareness, is able to sense the microbiota metabolites through its afferents, to transfer this gut information to the central nervous system where it is integrated in the central autonomic network, and then to generate an adapted or inappropriate response.”
“A cholinergic anti-inflammatory pathway has been described through VN's fibers, which is able to dampen peripheral inflammation and to decrease intestinal permeability, thus very probably modulating microbiota composition.”
“Stress inhibits the VN and has deleterious effects on the gastrointestinal tract and on the microbiota, and is involved in the pathophysiology of gastrointestinal disorders such as irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) which are both characterized by a dysbiosis. A low vagal tone has been described in IBD and IBS patients thus favoring peripheral inflammation. Targeting the VN, for example through VN stimulation which has anti-inflammatory properties, would be of interest to restore homeostasis in the microbiota-gut-brain axis.”
Some of this was confirmed by experiments which abused mice, such as those reported in the article “The Anxiolytic Effect of Bifidobacterium longum NCC3001 Involves Vagal Pathways for Gut–Brain Communication”. Essentially, the researchers gave the mice a poison which caused chronic colitis. The colitis in turn results in anxiety behaviours. However, if the mice had their Vagus Nerve severed first, the anxious behaviours were absent. This indicates the link between gut inflammation and brain inflammation (anxiety) gets communicated by the Vagus Nerve.
However, in those mice who had intact Vagus Nerves, and in which the colitis caused anxiety, when they were fed a beneficial gut bacteria, the anxiety was reduced. Finally, in mice who had established anxiety, then had their Vagus Nerves severed, then were given the probiotic, the bacteria did not reduce anxiety. So these results show that the Vagus Nerve is the communication pathway between the gut bacteria and the brain and is necessary for the gut microbiome to cause or reduce anxiety.
Connection to Neurotransmitters and Hormones
The gut flora and fauna may not only produce, in their waste products, many of the important chemical building blocks of our neurotransmitters, but also can eat them! So the endocrine system and the microbiome are also strongly bi-directionally linked.
Indeed, according to the article “Psychobiotics: How Gut Bacteria Mess with Your Mind”, gut bacteria can not only create neurotransmitters, but can also generate the fatty building blocks of what the brain itself is made from.
“These bacteria have a vast array of genes, capable of producing hundreds if not thousands of chemicals, many of which influence your brain. In fact, bacteria produce some of the same molecules as those used in brain signalling, such as dopamine, serotonin and gamma-aminobutyric acid (GABA). Furthermore, the brain is predominantly made of fats, and many of these fats are also produced by the metabolic activity of bacteria.”
Conversely, the article “Gut Bacteria Spotted Eating Brain Chemicals for the First Time” reveals that some gut bacteria survive by feeding on our neurotransmitters.
“Bacteria have been discovered in our guts that depend on one of our brain chemicals for survival. These bacteria consume GABA, a chemicak crucial for calming the brain, and the fact that they gobble it up could help explain why the gut microbiome seems to affect mood.”
“… ‘nothing made it grow, except GABA’. GABA acts by inhibiting signals from nerve cells, calming down the activity of the brain, so it’s surprising to learn that a gut bacterium needs it to grow and reproduce. Having abnormally low levels of GABA is linked to depression and mood disorders, and this finding adds to growing evidence that our gut bacteria may affect our brains.”
In terms of dopamine chemistry, gut microbiota have been found which can produce enzymes that block some of the chemical pathways, or eat l-dopa, a principal building block of dopamine. This is not good news for those of us with low baselines of dopamine!
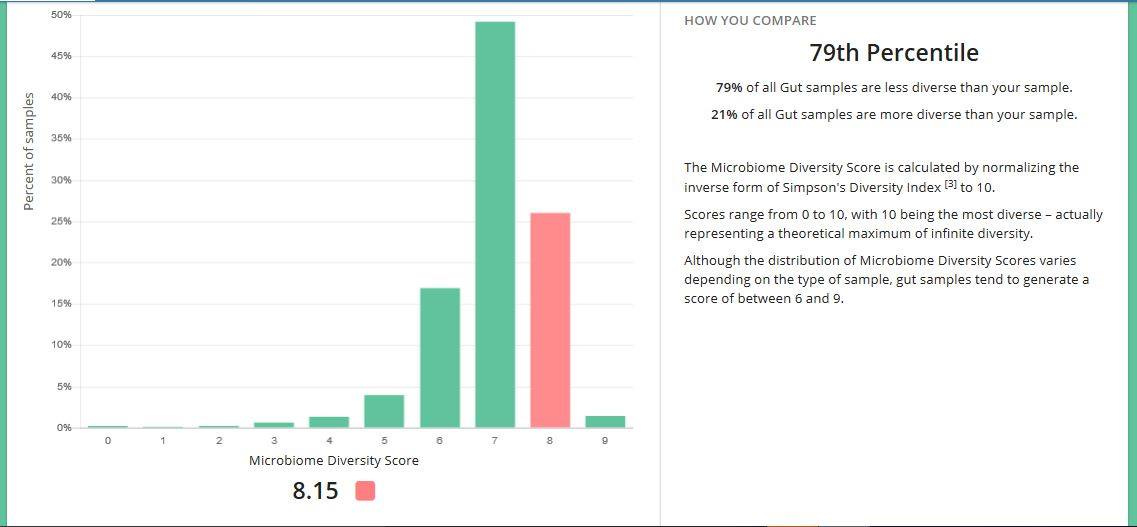
My Microbiome Test
Back in 2017, I got my gut microbiome analyzed by uBiome. In trying to interpret the results, I found some patent applications which seek to exploit previous findings from applying such analysis on people with Parkinson’s Disease
The application “Method for Diagnostics, Treatment and Prevention of Parkinson's Disease” states:
“… determining relative abundances of at least Prevotellaceae taxa [a type of bacteria] in the sample; and determining probability of the subject developing or having PD based on the abundances measured, wherein a high relative abundance of said taxa indicates a low probability of the subject developing or having PD.”
I checked my levels of Prevotella and it was trace amounts only: < 0.01%.
Furthermore, the patent states:
“determining the abundance of one or multiple of the following taxa:
Enterobacteriaceae, Clostridium XVIII, Anaerofilum, Papillibacter, Succiniclasticum, Klebsiella, Escherichia/Shigella and Paludibacter
in said sample, and determining by statistical methods the motor subtype based on the measured abundances, wherein low relative abundance of the said taxon or taxa indicates a tremor dominant subtype and high relative abundance indicates a non-tremor subtype.”
So I checked these too in my test report:
Enterobacteriaceae <0.01%
Clostridium 0.93%
Aneaerofilum <0.01%
Papillibacter: absent
Succinclasticum: absent
Klebsiella: absent
Eshrerichia: absent
Paludibacter: absent
So I would say I am definitely on the very low side of these, and again, there would seem to be abnormalities in these in people with PD. However, according to the patent above, I should have tremor dominant PD. Yet, I don't have tremors and never have.
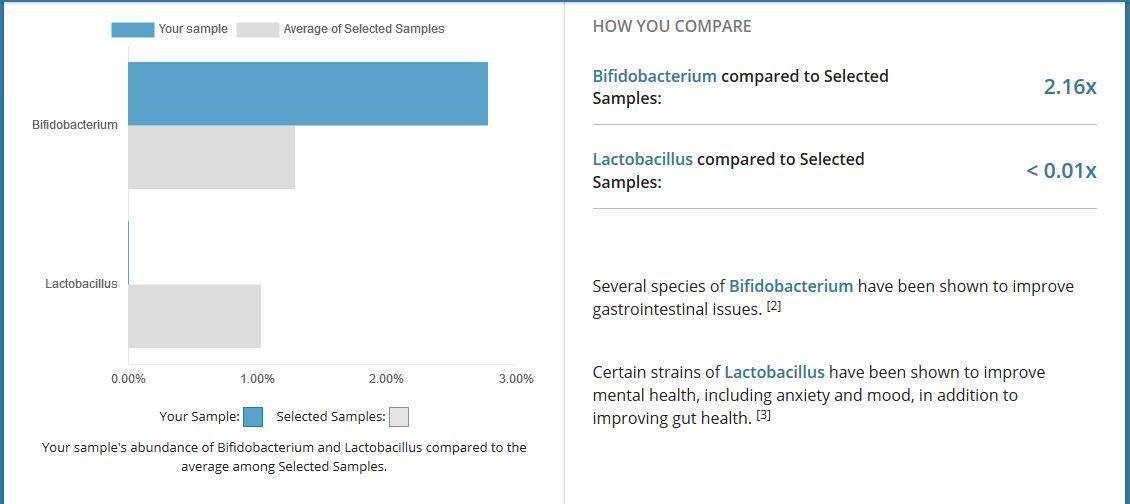
The test also showed up that I had almost none of the "good bacteria" of type lactobacillus. Why was this the case, and what wiped them out? And why since I'd been eating biolive goat's yoghurt twice a day for months before the test, which contains several strains of these, did they not come back? What was preventing them getting into my gut and staying alive?
Another interesting thing I learned through getting my gut bacteria characterized, is that body mass, and how easy it is too lose or put on weight, correlate with the types of gut bacteria we have. I was very lean and like many people with Parkinson's, found it hard to put or keep any weight on, no matter how much I ate [although this has changed recently, and I am beginning to be able to gain healthy weight]. My microbiome metrics on this showed that I had slightly more of the "lean" type bacteria than the average person.
I was surprised to learn that I had quite a diverse range of bacteria in my gut. I am wondering if this was due to the “good” (probiotic type) bacteria having “moved out”, or perhaps were actually never there, allowing room for lots of other bugs to move in and take up residence?
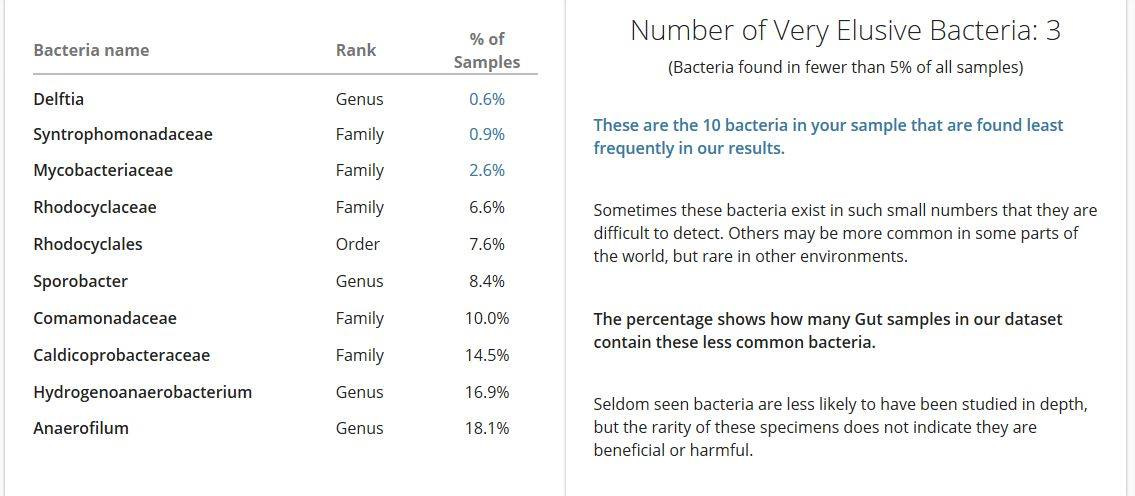
The analysis detected quite a few more weird and wonderful ones in my sample! I tried looking each of these up, but couldn't find much. Here are some things I did find:
Delftia acidovorans, a rare pathogen in immunocompetent and immunocompromised people;
Mycobacterium includes pathogens known to cause serious diseases in mammals, including tuberculosis (Mycobacterium tuberculosis) and leprosy (Mycobacterium leprae);
Rhodocyclaceae occur in waste water and play an important role in biological remediation in waste water treatment,




Lots of helpful information and source materials to ponder here. Thank you. Also, 'Threshold Theory', which makes perfect sense, could potentially be a good bridge term for getting some of those Germ Theorists and Terrain Theorists to talk to one another some day. Maybe.
Yes I already subscribe to Unglossed. I really enjoy his work. I also don’t completely deny the existence of “viruses.” From dissenting views I’ve read, they exist in that strands of RNA exist, but the impact they have of living systems and the mechanisms by which they have the theorized impacts are quite as clearly understood or even proven to the extent the proponents of viral theory would have us believe.
Thanks so much!